The classification of engineering materials
8 Feb , 2023
Engineering materials are generally classified into six major families (metals, polymers, elastomers, ceramics, glass, and composite hybrids), and they are split into metals, nonmetals, and hybrid materials. In this article, we explain in detail their main characteristics.
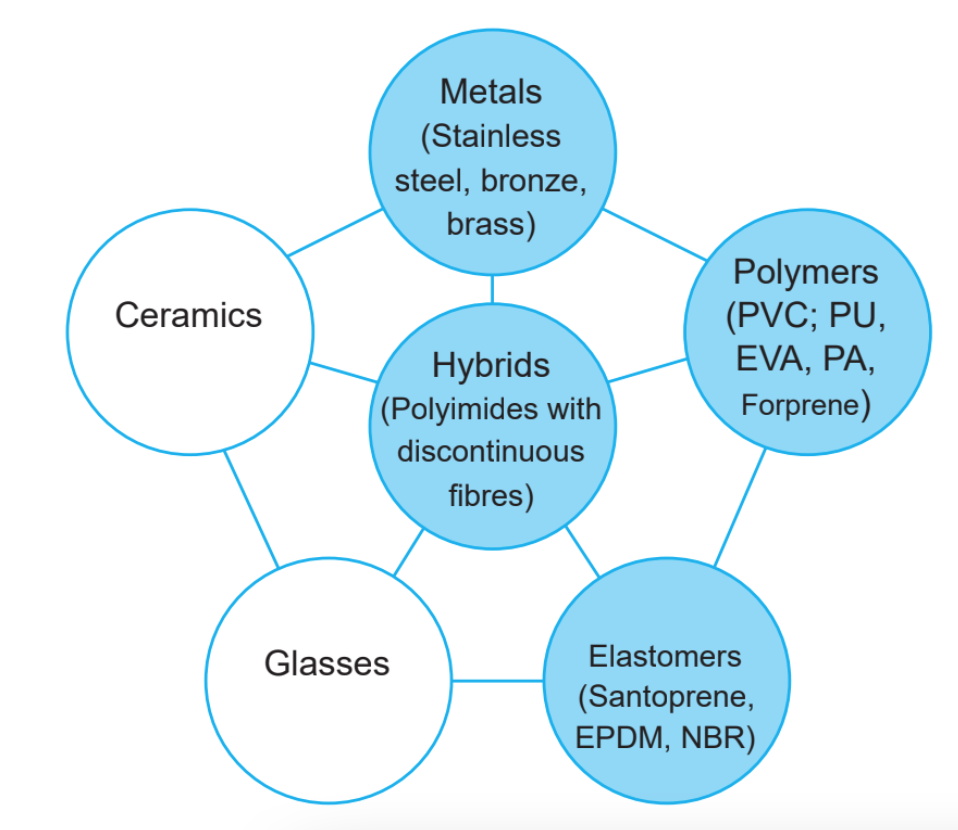
Metals
Metals have a high melting temperature, are good electrical and thermal conductors, and are hard and durable. Metallic materials are used in daily life for many applications and are split into two main categories: ferrous and nonferrous metals.
Ferrous metals
Ferrous metals contain iron and are magnetic, have low corrosion resistance (alloy steel with chromium content is used to avoid corrosion issues), and have good electrical and thermal conductivity.
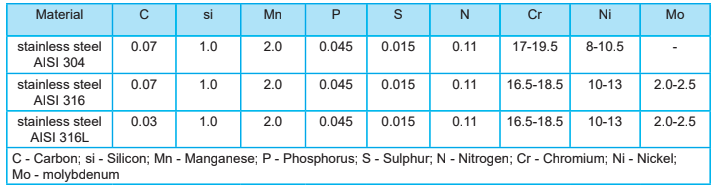
Examples of ferrous metals are low or mild-carbon steel, high-carbon steel, and cast iron. Stainless steel is naturally corrosion-resistant. AISI 304 stainless steel, AISI 316 stainless steel, AISI 316L stainless steel, and others are all examples of this.
The main difference between AISI 316 stainless steel and AISI 316L stainless steel is the carbon content. This type of steel alloy is used in very aggressive environments, such as in the naval sector, power plants, and explosive environments.
Nonferrous metals
Nonferrous metals do not contain iron and are non magnetic, have high corrosion resistance, and offer the best electrical and thermal conductivity. Examples of nonferrous metals are aluminum, copper, and silver. Examples of nonferrous metal alloys are brass (copper + zinc) and bronze (copper + aluminum + nickel).
Nonmetals
Nonmetals are materials with poor electrical and thermal conductivity and low melting temperature (there are some exceptions, however, such as diamond or graphite). Nonmetals focus on the following elements: oxygen (o), carbon (c), nitrogen (N), sulfur (s), chlorine (cl), and phosphorus (P).
The most common nonmetals are:
- thermoplastics (Polyurethane [PU], Polyvinyl chloride [PVC], Ethylene-vinyl acetate [EVA], Nylon [PA], Polypropylene [PP]) and thermosetting plastics (Polyimides)
- elastomers (EPDM, NBR, and santoprene)
- glass
- ceramics
Polymers consist of macromolecules, often in the form of large molecular chains in which the atoms are held together by covalent bonds, while the bonds between different chains are much weaker. For this reason, the molecular chain can be regarded as the basic unit of a polymer.
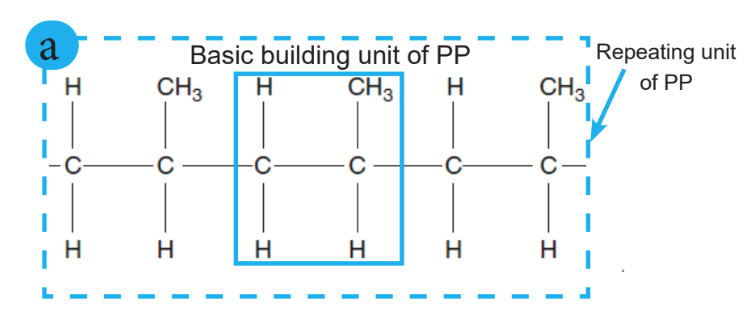
Linear chains are the units of polymers. However, it is possible to cross-link the chains covalently, forming a molecular network. These cross-links are crucial in determining the mechanical properties of the polymer because they fix the chains to each other and thus make it impossible to extract single-chain molecules.
In other words, polymerization is a reaction between monomer molecules in a chemical reaction to form polymer chains or three-dimensional networks.
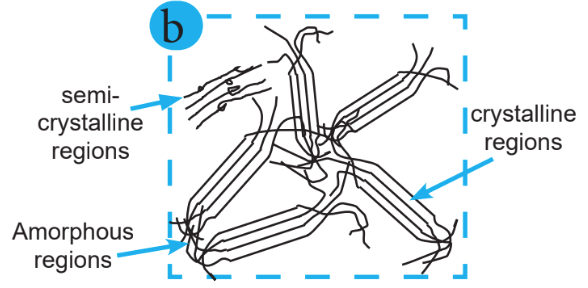
The polymer chains form a regular pattern, and the molecular structure forms a regular order of crystals, which is known as the crystalline region. Polymer chains are randomly arranged, and the molecular structure that is unable to form a regular order with the molecules is known as the amorphous region. The mix of the crystalline region and amorphous region is known as the semicrystalline region.
Hybrid materials
An example of a hybrid material is the mixing of polyamides (thermosetting) with discontinuous fibers and the manufacturing of products in injection molding. In addition, flexible pipes in steel or stainless steel AISI 304 or stainless steel AISI 316L in combination with recovered plastic material (PVC or EVA or PU, or Forprene) are considered made in a hybrid material. They are also known as composite conduits..
Feel free to download a detailed document here with the classification of materials, polymers, and information on the applications of the various materials used to create Euro 2000 products.

